Bioluminescence: Chemical Study on Visible Light Emission from Fungal Mycelium and Fruiting Body - Juniper Publishers
Journal of Trends in Technical and Scientific Research
Abstract
Although the visible light emission by living
organisms is generally thought to be rare, in fact the bioluminescent
phenomenon can be widely observed in nature, for examples insects,
fishes, bacteria, and fungi. The phenomenon has intrigued scientific
researchers over the years.The chemical mechanisms underlying
bioluminescence differ between species. Some mechanisms, such as
bioluminescence of firefly, jellyfish, bacteria, and dinoflagellate,
have been elucidated and further more their principles have allowed the
development of many novel technologies in agriculture, biology, ecology,
and medicine. However, many other bioluminescence mechanisms yet remain
to be understood. Further research of bioluminescence will lead
discoveries of biological significance and novel possibility in science.
Here I present studies on chemical mechanism underlying fungal
bioluminescence, which are actively in progress.
Keywords: Bioluminescence; Chemiluminescence; Fungus; Luciferin; Luciferase; Mechanism
Mini Review
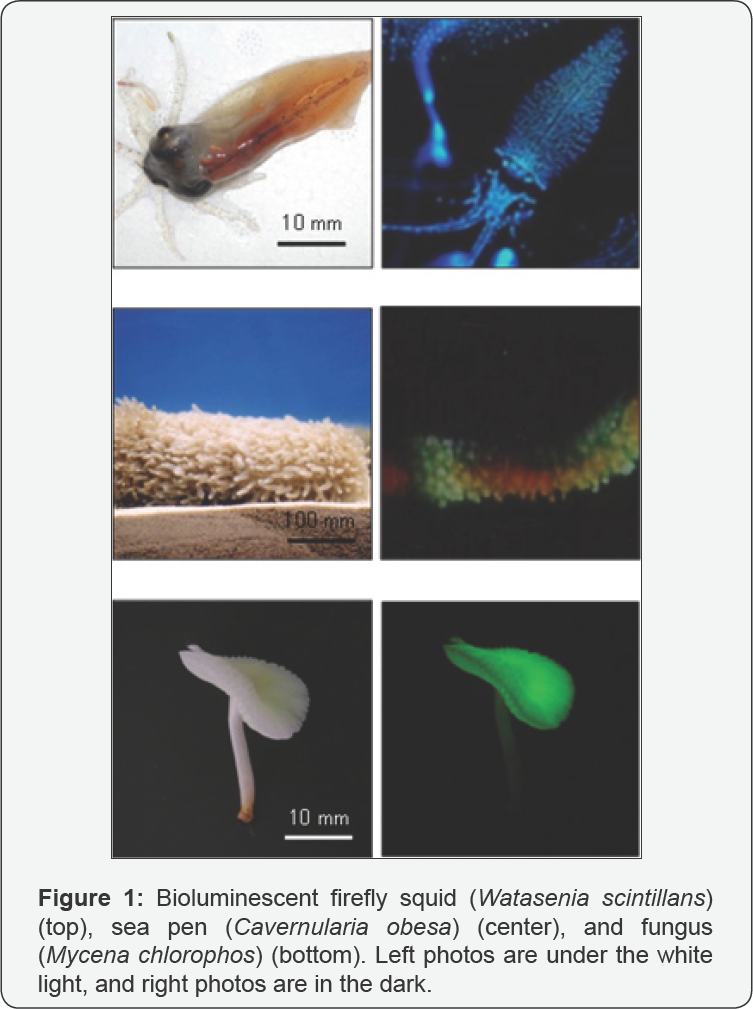
Bioluminescence is the emission of visible light by
living organisms, such as bacteria, fireflies, fishes, ostracods of the
genus Cypridina, and jellyfish of the genus Aequorea [1]. The bioluminescence phenomenon has been arousing fascination in human and curiosity in scientists from a long time ago (Figure 1).
Detailed chemical principles in bioluminescence have been disclosed by
biological, organic, and physical chemists since 1950's, and then it has
been understood that the bioluminescence phenomenon is caused by energy
conversion of chemical energy to light energy via chemical reactions in
bioluminescence systems. Moreover, the bioluminescence principles have
been applied in many scientific fields such as agriculture, biology,
ecology, and medicine [2].
Since instrumentation for measuring light is very sensitive and low
background, chemiluminescence methods based on the bioluminescence
principles has come into widespread use for quantitative determination
of specific substances in many scientific fields. Bioluminescent
organisms use their light, which possesses various colors, periodic
patterns, and intensities, for self-defense against predators,
camouflage, intra specific communication, or attracting mates or prey [3].
Fungal bioluminescence is also widely observed on
decaying wood or leaves in night. Thus, the phenomenon is called
“foxfire” or "shining wood”. The total number of documented species of
luminous fungi by 2016 is more than 80 [4]. Fungal bioluminescence, which varies among species, occurs in the basidiocarp, mycelium, spores, or in
Trends Tech Sci Res 1(3): TTSR.MS.ID.555565 (2018) their combinations [5-7].
Although fungal bioluminescence generally shows lower intensities than
other bioluminescent organisms such as fireflies and ostracods, the
light emission from fungi is continuously generated for day and night.
Thus, total light emission from certain bioluminescent fungi may be
comparable with that of firefly. The color of fungal bioluminescence is
commonly green. With regard to functions of fungal bioluminescence,
Olivera et al. reported that fruiting bodies of bioluminescent fungus Neonothopanus gardneri can attract potential spore dispersing insects by glowing [8]. On the other hand, Weinstein et al. reported that Australian fruiting bodies of bioluminescent fungus Omphalotus nidiformis did not attract the insects [9].
The mycelium is underground or inside a decaying wood. It is possible
that fungal bioluminescence is a by-product of metabolism, and possesses
no function. Functions of fungal bioluminescence yet remain to be
understood.
In spite of the wide popularity of fungal
bioluminescence, chemical mechanism underlying fungal bioluminescence
has been subjected to far less scientific investigation than the
bioluminescence from other sources. In 1668, Boyle reported that air was
needed for the light emission of shining wood [10].
Although many researchers studied on chemical mechanism underlying
fungal bioluminescence, their efforts were unsuccessful until Airth's
group showed positive results using bioluminescent fungi Collybia velutipes and Armillariamelleain 1959 [11].
Airth et al. reported that the bioluminescence was NAD(P)H-dependent
luminescence reaction, in which unknown substrate (luciferin), molecular
oxygen, water-soluble enzyme, and water-insoluble enzyme (luciferase)
were involved [1113].
However, after that, the luciferase and luciferin were not isolated and
their structures and luminescent properties were not determined.
Kuwabara and Wassink isolated a luciferin, which displayed
chemiluminescence in the presence of H2O2, in a crystal form from the mycelia of Omphalia flavida [14]; however, its chemical structure was not reported.
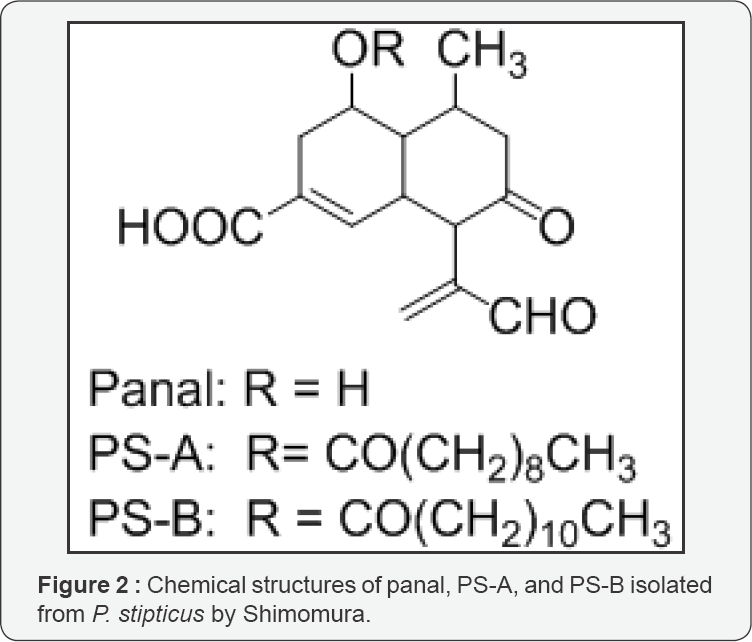
Shimomura demonstrated a negative result with regard
to the Airth's luciferin-luciferase reaction to the fruiting body
bioluminescence of the luminous fungus Panellus stipticus; the precursors, panal, PS-A, and PS-B (Figure 2),
of light-emitting compounds were isolated from the luminous fruiting
bodies that upon activation with ammonium or primary amines, emitted
light in the presence of H2O2 and Fe2+ or in the presence of superoxide anion and molecular oxygen [15,16].
Moreover, Shimomura purified the chemiluminescent activation products
that were produced by reaction of the precursors with methylamine, and
inferred the possible structures of the activation products using the
model compounds of the precursors [17]. These activation products or compounds corresponding to the activation products in the P. stipticus
fruiting bodies have not yet been found. Subsequently, Shimomura
analyzed the interrelationships between the bioluminescence and the
superoxide dismutase activities using six species of fungi, including P. stipticus (fruiting body and mycelium), and demonstrated that superoxide dismutase regulated the bioluminescence activity [18]. On the basis of these findings, Shimomura suggested that, unlike Airth's luciferin-luciferase reaction, P. stipticus
bioluminescence was not a luciferin-luciferase type. Shimomura also
suggested that superoxide anion triggered the bioluminescence reaction
in the presence of molecular oxygen for generating light.

In 2012, Oliveira et al. reported the hypothesis that
all known bioluminescent fungal lineages share luciferin- luciferase
reaction shown by Airth's group [19].
In 2015, Yampolsky's group reported that and trans-3-hydroxyhispidin,
which played as luciferin with water-insoluble luciferase, was produced
from trans-hispidin with molecular oxygen and NADPH in the presence of
water-soluble hydroxylase for mycelia bioluminescence of Neonothopanus nambi (Figure 3) [20].
Then, Yampolsky's group reported that and trans-3-hydroxyhispidin was
oxidized to (2Z,5E)-6-(3,4-dihydroxyphenyl)-2-hydroxy-
4-oxohexa-2,5-dienoic acid(oxyluciferin) as a light emitter in the
presence of a luciferase-enriched protein fraction prepared from N. gardneri and N.nambi mycelium (Figure 3) [21]. Trans - 3-hydroxyhispidin is a strong “candidate” of luciferin in N. gardneri and N.nambi mycelia at the present time. Luciferase has been successfully extracted from N. gardneri and N.nambi mycelia and partially purified [21].
Therefore, it is distinct that these mycelia possess luminescence
systems involving trans-3-hydroxyhispidin. In the future it is necessary
to elucidate whether these luminescence systems generate true
bioluminescence in the living N. gardneri and N.nambi
mycelia or not, and whether similar bioluminescence systems involving
trans-3-hydroxyhispidin exist in other bioluminescent fungi or not.
Although various fungal species have been used for
chemical research of fungal bioluminescence over the years, mycelia were
subject for the research, except for Shimomura's experiments above
mentioned. Some researchers believe that bioluminescence mechanism for
all bioluminescent fungi should be the same, and that chemical mechanism
behind bioluminescence in mycelia and fruiting bodies is the same.
Because bioluminescent fungus Mycena chlorophos (Figure 1), which was found in Southeast Asia [22] and can be cultivated in laboratory [23,24], produces continuously bright light in the mycelium and pileus gills, in which light is produced in cell membrane [25],
this species is a suitable for evaluating those hypotheses. Teranishi
has been chemically investigating bioluminescence mechanism of this
fungus using living tissue. Generally when fungal tissue is crushed,
bioluminescence is immediately stopped. Because the crushed tissue
losses the bioluminescence ability, the crushed tissue and extracts from
the tissue cannot be used for luminescence assays. Recently Teranishi
demonstrated that trans-4-hydroxycinnamic acid and
trans-3,4-dihydroxycinnnamic acid in gills increased the bioluminescence
intensity in living immature gills of M. chlorophos, which emitted weak light [26,27], and that other cinnamic acids shown in Figure 4
did not influence the original light emission. Moreover, the light
emission before and after addition of trans-4-hydroxycinnamic acid
ortrans-3,4-dihydroxycinnnamic acid to gills was inhibited by addition
of trans-4-aminocinnnamic acid, whereas trans-2- and trans-3-aminocinnnamic acids did not inhibit the light emission [28].These results indicate that trans-4- and/or 3,4-di-hydroxycinnamic acids contribute to bioluminescence in M. Chlorophos living gills, and that the hydroxy group at position C-4 intrans-cinnamic
acid plays an important role for producing the energy for light
emission. Although Teranishi reported that the combination of NADPH and
hispid in was not essential for the production of bright light in the
living gills [29],
bioluminescence ability of trans-3-hydroxyhispidin yet remains to be
elucidated. Investigation for understanding the chemical mechanism
underlying bioluminescence observed in M. chlorophos gills and mycelia is in progress.

To Know More About Trends in Technical and ScientificResearch click on: https://juniperpublishers.com/ttsr/index.php
To Know More About Open Access Journals Please click on:



Comments
Post a Comment